Order MSCs
High-quality MSCs From our Facility in Stockholm
We offer GMP-grade and research-grade allogeneic mesenchymal stem cells (MSCs) for clinical and preclinical applications. We also produce custom-made MSC products from various sources.
The cell production process is based on 25 years of research and clinical experience at the Karolinska Institute. Our large-scale facility allows for high quality at competitive prices.
Our manufacturing is EU-GMP approved by the Swedish Medical Products Agency and we deliver to clinical trials around the world.
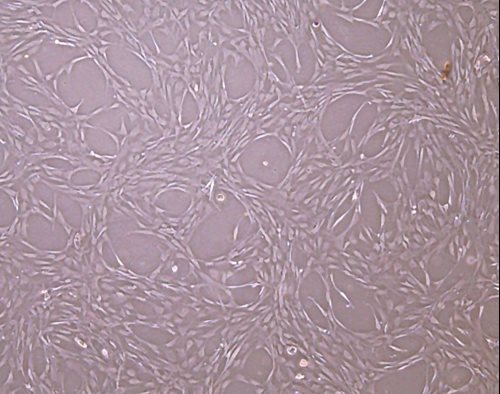
Research-Grade MSCs
- 1 or 10 million cells per vial
- Bone marrow or adipose derived
- Xeno-free
- Passage 2 or 3
- International delivery in dry ice
GMP-grade MSCs
- Adjustable amount and vial size per dose
- Bone marrow or adipose derived
- Xeno-free
- Passage 2 or 3
- International delivery in liquid nitrogen
Please contact us for more information
Product Information
- Safety Data Sheet - Research Grade Mesenchymal Stem Cells (PDF)
- Product Sheet - Research Grade Mesenchymal Stem Cells (PDF)
- Terms and Conditions (PDF)
- GMP Compliance Certificate (PDF)
FAQ
Publications by Katarina Le Blanc
Our Distributors
BIOZOL offers more than 25 million products from over 260 partner companies worldwide. More than 14,000 satisfied customers from universities, research institutes, and pharmaceutical and biotech companies have benefited from BIOZOL's services for more than 30 years.
2BScientific is a leading distributor of innovative life science products, serving customers in the UK, Europe, and worldwide. Our top priority is always the customer, and we go above and beyond to provide exceptional service and innovative products that support scientific discovery.
CliniSciences commercializes reagents to diagnostic and research labs. As a very dynamic company, we do our best to be close to our customers' needs in terms of the high-quality reagents that we propose and in terms of the service that we provide. Our principal assets for better serving you are adaptability, flexibility, reactivity, and the competence of the technical specialists at your service.
Atlantis Bioscience is a Singapore-based distributor committed to helping scientists and researchers achieve new heights in their projects by distributing the most cutting-edge research solutions on a global scale. We strive to provide a one-stop-shop for high-quality products and services, delivering globally to Asia, Europe, North America, Oceania, and South America.
Based in Massachusetts, ARP has been providing high-quality products to research laboratories in hospitals, universities and biotech firms worldwide since 1994. Our commitment to detail and quality will ensure the results you have come to expect.

PELO biotech offer special Cell Culture Media for Human Primary Cells, Stem Cells and Embryonic Stem Cells as well as special supplements for Tumor Stem Cells. Their mission is to provide high-quality cell culture products at affordable prices.
Looking for Treatments?
Cellcolabs does not provide treatments. However, we can provide updates on how and when it is possible to join clinical trials using our MSCs.


.pngg?width=307&height=135&mode=max)
